
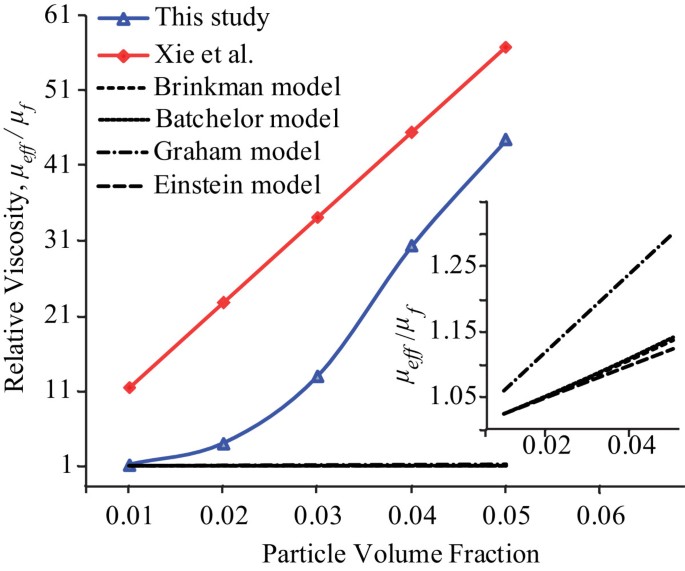
K) at 25 ☌ – the second-highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules.Water has a very high specific heat capacity of 4184 J/(kg
Heat of vaporization of water from melting to critical temperature Heat capacity and heats of vaporization and fusion This pressure is reached at a depth of about 2200 meters: much less than the mean depth of the ocean (3800 meters). A likely example of naturally occurring supercritical water is in the hottest parts of deep water hydrothermal vents, in which water is heated to the critical temperature by volcanic plumes and the critical pressure is caused by the weight of the ocean at the extreme depths where the vents are located. In nature this only rarely occurs in extremely hostile conditions. The critical temperature is 647 K and the critical pressure is 22.064 MPa. Visible steam and clouds are formed from minute droplets of water suspended in the air. The gaseous phase of water is known as water vapor (or steam). Aside from common hexagonal crystalline ice, other crystalline and amorphous phases of ice are known. The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular crystals, like snow. Within the Earth's atmosphere and surface, the liquid phase is the most common and is the form that is generally denoted by the word "water". However, these bonds are strong enough to create many of the peculiar properties of water, some of which make it integral to life. The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2 × 10 −13 seconds). This unique property of water is due to hydrogen bonding. Under standard conditions, water is primarily a liquid, unlike other analogous hydrides of the oxygen family, which are generally gaseous. Large ice crystals, as in glaciers, also appear blue. This can easily be observed in a water-filled bath or wash-basin whose lining is white. Liquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Water is a tasteless, odorless liquid at ambient temperature and pressure. Water is the chemical substance with chemical formula HĢO one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Related to its amphoteric character, it undergoes self-ionization. Water is amphoteric, meaning that it can exhibit properties of an acid or a base, depending on the pH of the solution that it is in it readily produces both H + Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 ☌ for its molar mass, and a high heat capacity. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Water molecules form hydrogen bonds with each other and are strongly polar. It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide).

It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life." It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. 2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue.


 0 kommentar(er)
0 kommentar(er)
